This post is the Second Part of Methods of sterilization.
This is the continuation of Methods of sterilization part 1.
Till now we have learned about physical methods of sterilization.
In this post we will move further towards mechanical and chemical methods of sterilization.
Table of Contents
ToggleMECHANICAL METHODS OF STERILIZATION
FILTRATION
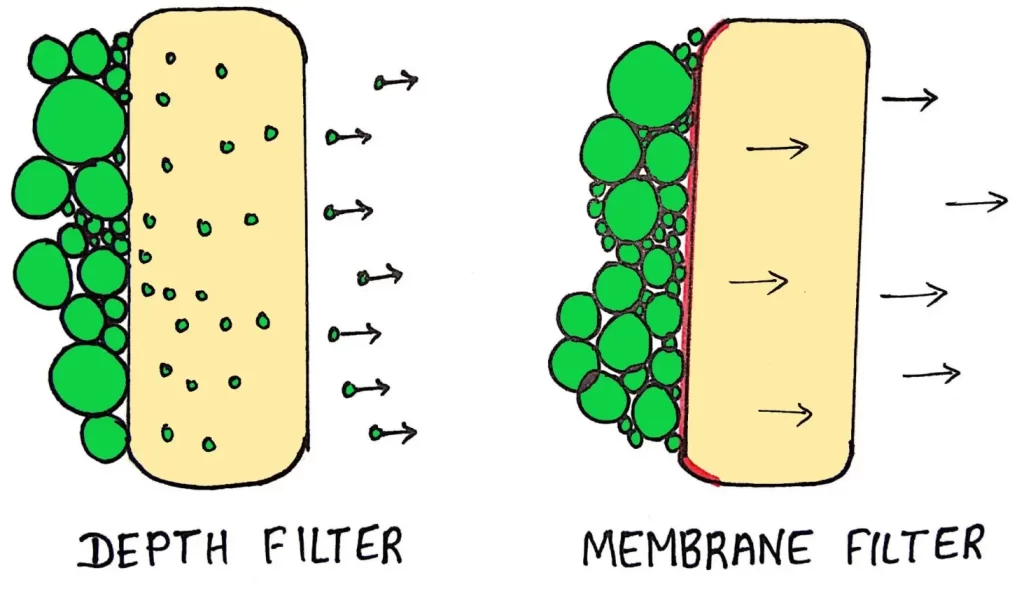
- Filtration is the process in which liquid or gas is passed through a screen with pores.
- Screen separates bacteria from suspension.
- Pores are smaller enough to retain microorganisms.
- Used to sterilize those material which may get damaged or denatured by chemicals or high temperature.
Two types:
- Depth filters- Retains microbes throughout the depth of filter rather than on surface.
- Membrane filters- Retains microbes larger than the pore size on the surface.
Uses:
- To separate bacteriophage, toxins and viruses.
- To sterilize area, sugar and antibiotic solutions.
- To purify air or water.
CHEMICAL METHODS OF STERILIZATION
1. ALCOHOLS
- Alcohols denatures Proteins, obstructs membranes and breaks down many lipids.
- They rapidly evaporate after action without leaving any traces.
- Product should be rubbed on the surface of hands and fingers until they dry.
- Not good for open wounds because they can coagulate Protein layer under which microbes can grow.
- Commonly used alcohols are- ETHANOL, ISOPROPANOL etc.
- Isopropanol is more effective, less volatile, less expensive and more easily available commercially.
- Alcohol can kill bacteria and fungi but not endospores.
2. ALDEHYDES
- One of the most effective antimicrobial chemical agents.
- Commonly used aldehydes are- FORMALDEHYDE, GLUTARALDEHYDE etc.
- They act by inactivation of Protein.
- Commercially Formaldehyde is available as Formalin having 37% formaldehyde gas which is an excellent disinfectant.
- Glyceraldehyde is less irritant and more effective compared to Formaldehyde. So, it is used to sanitize hospital equipments.
- Both are used for Embalming (preservation of corpse of human or animal body or their parts from decomposition for many years).
3. PHENOLS
- In 1867, phenols were first time used by Joseph Lister (father of antiseptic surgery).
- Derivatives of phenols are called phenolics which are used as disinfectant in hospitals and laboratories.
Examples- Lysol, Cresol, Xylenol etc.
- Acts by damaging lipids on plasma membrane.
- They are stable, remain active in presence of organic compounds, can remain for long time after application.
- They can control the odour in sewage.
- They are toxic, irritant to skin, unpleasant odour so, rarely used.
- Few phenolics are less irritant and remain on skin for long time.
Chlorhexidine- Used in Savlon.
Chloroxylenol- Used in Dettol.
4. HALOGENS

- IODINE and CHLORINE are most effective disinfectants.
- They remain in free state, can form salt with sodium and other metals.
- Iodine is most effective as it can kill all the species of bacteria, endospores, fungi, and some species of viruses too.
- Iodine impairs synthesis of Protein and alters the cell membranes of microorganisms.
- Iodine is available in tincture form (solution in aqueous alcohol).
Example: Betadine.
- Iodine are used to disinfect skin and wounds.
- Chloride compounds are also very effective disinfectants.
Example:
- Sodium hydroxide is a domestic disinfectant and Bleach.It is used in dairies, food industries and haemodialysis systems.
2 drops of Bleach in 1 liter of water (4 drops in cloudy water) makes drinking water safe.
5. OXIDIZING AGENTS
- HYDROXY PEROXIDE and PERACETIC ACID are commonly used agents.
- Hydroxy peroxide is a strong oxidizing agent.
- Attacks membrane, lipids, DNA and other cellular components of microorganisms.
- Used to disinfect ventilator, contact lenses, tonometer etc.
- Safe, non-toxic and non-carcinogenic.
- Peracetic acid is more active than hydroxy peroxide.
- Used to disinfect hemodialyzers and plasma sterilization.
- But it can corrode steel, iron, copper, bronze and brass.
6. HEAVY METALS

- Heavy metal salts like MERCURY, SILVER, ARSENIC, ZINC and COPPER are widely used from centuries due to their germicidal effects.
- Egyptians discovered that putting silver coin in barrels of water can keep water clean from harmful microbial growths.
- These metals can combine with Proteins of bacterial cell and inactivate them so, they act as bacteriostatic (not bactericidal).
- Examples:
- Silver sulphadiazine used in burn patients.
- Silver nitrate 1% solution were used in infants to prevent eye infection.
- Copper sulphate works as a fungicide in lakes and swimming pools.
- Mercury salts are known antiseptics and antifungal agents but, they are not used now a days. Thiomersal is used as a preventive for vaccines, sera or immunoglobulin preparations.
7. SURFACE ACTIVE AGENTS/ SURFACTANTS
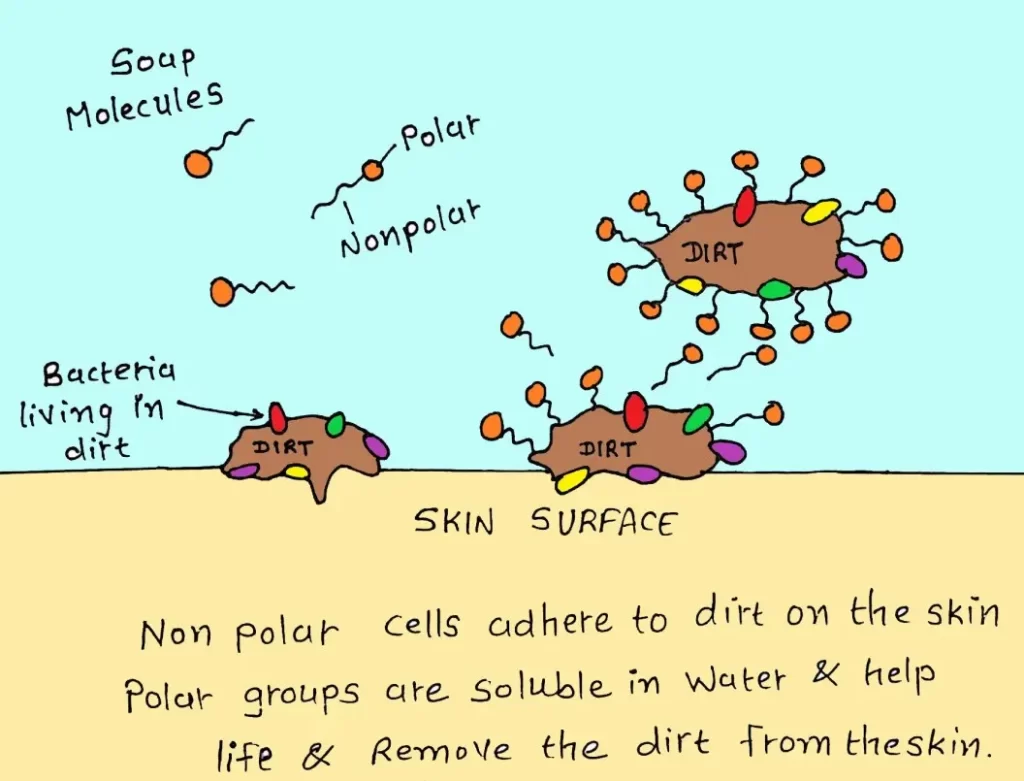
- They can decrease the surface tension between liquid molecules.
Examples: SOAPS and DETERGENTS.
- They do not act as an antiseptic but can remove microbes mechanically through scrubbing.
- Dead cells, dust, dried sweat and other oily glandular secretions on the skin can be washed off with the help of soap.
- Soap forms tiny droplets of oily secretions (emulsification) then water and soap together lift up these droplets and washes them away.
- Washing hands with soap and water is an effective sanitization method.
- They are stable, non-harmful to skin.
- Inactivated by acidic pH, organic matter, hard water and soap.
Use warm water and soap, rub them with hands together for at least 20 seconds, then rinse.
Dry with a paper towel or air dryer, turn off the faucet using paper towel.
8. DYES
- Dyes like ANILIN & ACRIDINE are widely used as skin and wound antiseptic.
- Aniline dyes mostly affect Gram positive bacteria.
- Non-toxic, non-irritant.
- Becomes inactive in presence of organic compounds.
- They affect synthesis of peptidoglycan layer of bacterial cell.
- Used as selective agent in culture media in laboratories.
Examples: Gentian violet, Malachite crystal violet, Brilliant green.
- Acridine dyes are less affected by organic compounds.
- More affects Gram positive bacteria but not as selective as aniline dyes.
- They interrupt Protein and Nucleic acid synthesis in bacterial cell.
Examples: Acriflavine, Euflavine, Proflavine, Aminacrine etc.
9. GASEOUS STERILIZATION
- Widely used gaseous sterilizer is Ethylene oxide (EtO).
- Kills both vegetative and spore forms of bacteria.
- Acts by combining and denaturing the cellular Proteins.
- Very inflammable, irritant, explosive and carcinogenic that’s why not suitable for fumigation (a process of killing microbes by using lethal gas, vapours or smoke).
- A special instrument known as Ethyne oxide sterilizer is used.
- Used to sterilize heat sensitive equipments like plastic petri dishes, syringes, sutures, catheters, respiratory and dental utensils.